Page 7 - qp_17
P. 7
MAKMAL KESIHATAN AWAM KOTA BHARU
Document Number : MKAKB/BP/QP-17
QUALITY PROCEDURE
Issue Number : 01
METHOD SELECTION, VALIDATION & Issue Date : 07.06.2018
VERIFICATION Page Number : 4 of 7
NO PROCEDURE RESPONSIBILITY
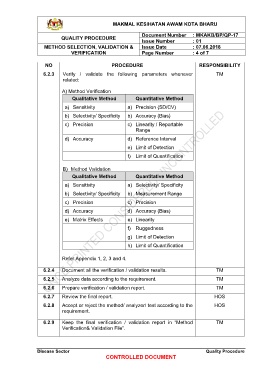
6.2.3 Verify / validate the following parameters whenever TM
related:
A) Method Verification
Qualitative Method Quantitative Method
a) Sensitivity a) Precision (SD/CV)
b) Selectivity/ Specificity b) Accuracy (Bias)
c) Precision c) Linearity / Reportable
Range
d) Accuracy d) Reference Interval
e) Limit of Detection
f) Limit of Quantification
B) Method Validation
Qualitative Method Quantitative Method
a) Sensitivity a) Selectivity/ Specificity
b) Selectivity/ Specificity b) Measurement Range
c) Precision c) Precision
d) Accuracy d) Accuracy (Bias)
e) Matrix Effects e) Linearity
f) Ruggedness
g) Limit of Detection
h) Limit of Quantification
Refer Appendix 1, 2, 3 and 4.
6.2.4 Document all the verification / validation results. TM
6.2.5 Analyze data according to the requirement. TM
6.2.6 Prepare verification / validation report. TM
6.2.7 Review the final report. HOS
6.2.8 Accept or reject the method/ analyzer/ test according to the HOS
requirement.
6.2.9 Keep the final verification / validation report in “Method TM
Verification& Validation File”.
Disease Sector Quality Procedure
CONTROLLED DOCUMENT